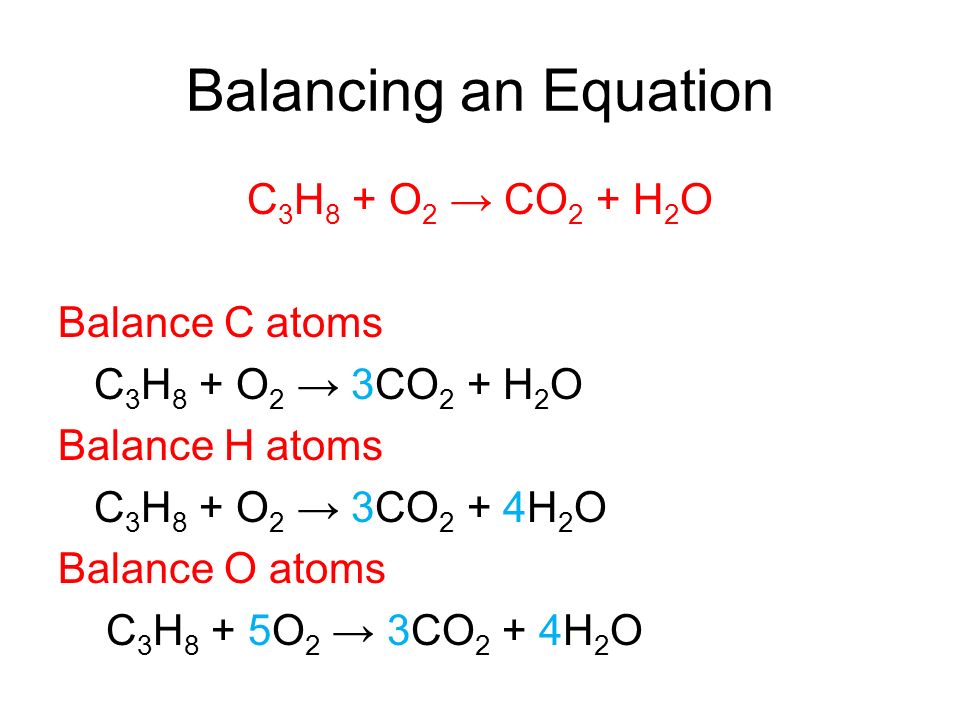
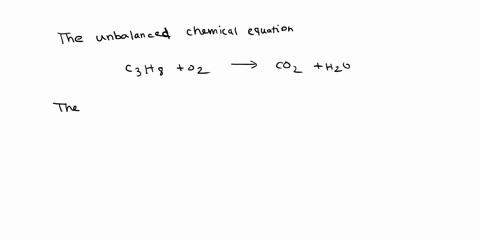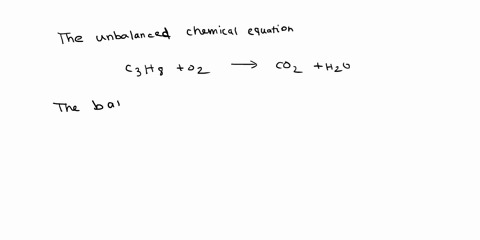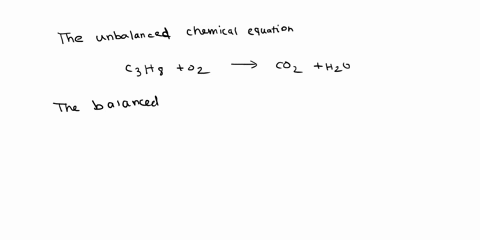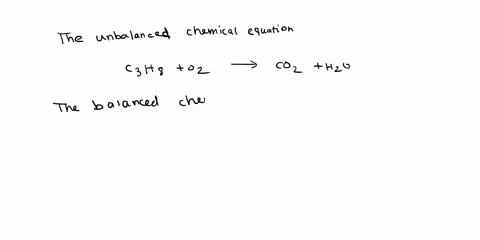
Given the following reaction: C3H8 + O2 → CO2 + H2O, what is the number of moles of carbon dioxide produced? If you start with 14.8 g of C3h8 and 3.44 g

SOLVED: Consider the following equation: C3H8 + O2 = CO2 + H2O, which underwent a combustion reaction of 2.20 g C3H8 with excess O2. How many grams of carbon dioxide were formed?

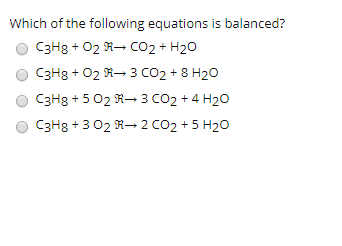
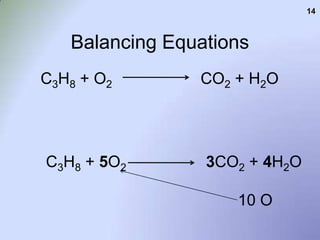

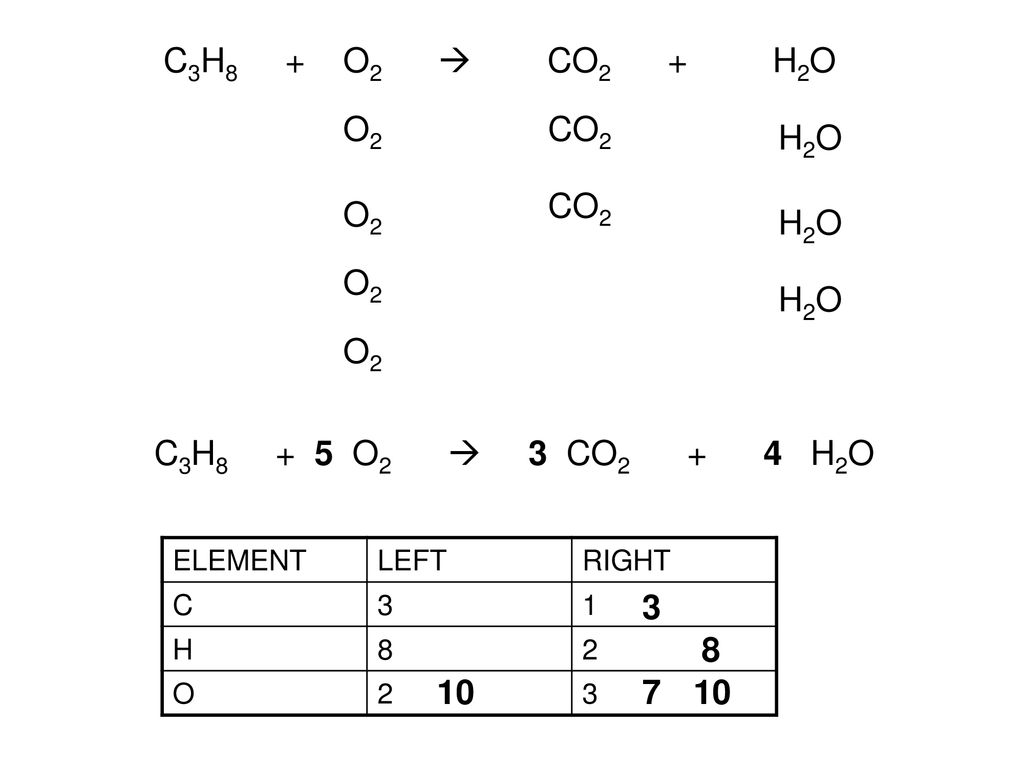





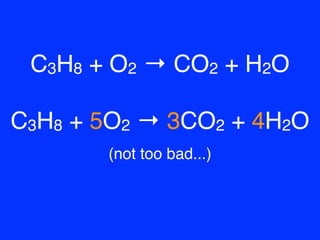

![C3H8 + O2 = CO2 + H2O, Balance the equation [Step by Step] C3H8 + O2 = CO2 + H2O, Balance the equation [Step by Step]](https://topblogtenz.com/wp-content/uploads/2023/01/c3h8-o2-co2-h2o-balancing-equation-min.png)