Health Canada approves Zolgensma®, the one-time gene therapy for pediatric patients with spinal muscular atrophy (SMA)
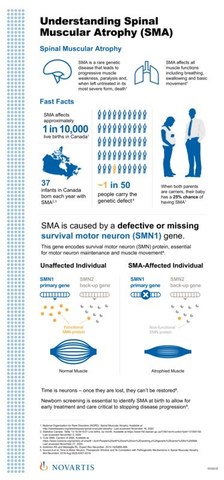
Health Canada approves Zolgensma®, the one-time gene therapy for pediatric patients with spinal muscular atrophy (SMA)
FDA Lifts Partial Clinical Hold on Novartis' Gene Therapy Trial for Spinal Muscular Atrophy | BioSpace


/cloudfront-us-east-2.images.arcpublishing.com/reuters/T6XZTIM52JONFIX43C4EHTWNTQ.jpg)